Feb 21 2013
University of California, San Diego bioengineers have demonstrated in a study in pigs that a new injectable hydrogel can repair damage from heart attacks, help the heart grow new tissue and blood vessels, and get the heart moving closer to how a healthy heart should. The results of the study were published Feb. 20 in Science Translational Medicine and clear the way for clinical trials to begin this year in Europe. The gel is injected through a catheter without requiring surgery or general anesthesia -- a less invasive procedure for patients.
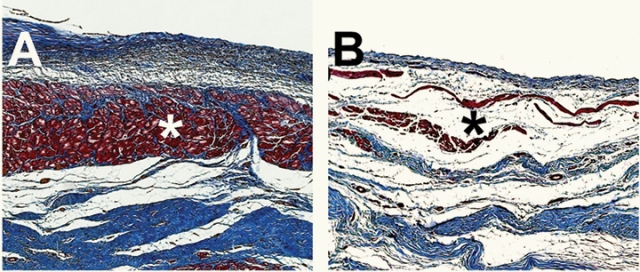 Microscopic images of pig hearts damaged by heart attack show the growth of new heart muscle tissue (Shown in Red, Figure A) after treatment with an injectable hydrogel compared to a heart left untreated (Figure B, right). Photo credit: Karen Christman, UC San Diego Jacobs School of Engineering.
Microscopic images of pig hearts damaged by heart attack show the growth of new heart muscle tissue (Shown in Red, Figure A) after treatment with an injectable hydrogel compared to a heart left untreated (Figure B, right). Photo credit: Karen Christman, UC San Diego Jacobs School of Engineering.
There are an estimated 785,000 new heart attack cases in the United States each year, with no established treatment for repairing the resulting damage to cardiac tissue. Lead researcher Karen Christman, a professor in the Department of Bioengineering at the UC San Diego Jacobs School of Engineering, said the gel forms a scaffold in damaged areas of the heart, encouraging new cell growth and repair. Because the gel is made from heart tissue taken from pigs, the damaged heart responds positively, creating a harmonious environment for rebuilding, rather than setting off a chain of adverse immune system defenses.
Karen Christman decellularized extracellular matrix video
“While more people today are initially surviving heart attacks, many will eventually go into heart failure,” said Christman. “Our data show that this hydrogel can increase cardiac muscle and reduce scar tissue in the region damaged by the heart attack, which prevents heart failure. These results suggest this may be a novel minimally invasive therapy to prevent heart failure after a heart attack in humans.”
The hydrogel is made from cardiac connective tissue that is stripped of heart muscle cells through a cleansing process, freeze-dried and milled into powder form, and then liquefied into a fluid that can be easily injected into the heart. Once it hits body temperature, the liquid turns into a semi-solid, porous gel that encourages cells to repopulate areas of damaged cardiac tissue and to improve heart function, according to Christman. The material is also biocompatible; animals treated with the hydrogel suffered no adverse affects such as inflammation, lesions or arrhythmic heart beating, according to safety experiments conducted as part of the study. Further tests with human blood samples showed that the gel had no affect on the blood’s clotting ability, which underscores the biocompatibility of the treatment for use in humans.
San Diego-based startup, Ventrix, Inc., which Christman co-founded, has licensed the technology for development and commercialization. Christman also serves on the company’s board. “We are excited and encouraged by the results of the study leading to a novel regenerative medicine solution for cardiac repair. The technology offers the potential for a longer and better quality of life for millions of heart attack sufferers,” said Adam Kinsey, the CEO of Ventrix.