Jun 18 2013
Researchers from Ulsan National Institute of Science and Technology (UNIST), S. Korea, developed a novel, simple method to synthesize hierarchically nanoporous frameworks of nanocrystalline metal oxides such as magnesia and ceria by the thermal conversion of well-designed metal-organic frameworks (MOFs).
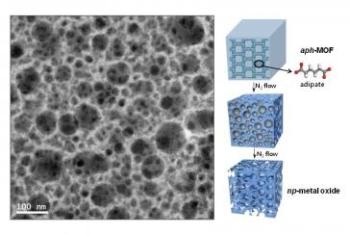 Shown at left is a TEM image of np-MgO-500. At the right is the schematic view of the direct conversion from aph-MOG to np-metal oxide by heating under nitrogen atmosphere. Credit: UNIST
Shown at left is a TEM image of np-MgO-500. At the right is the schematic view of the direct conversion from aph-MOG to np-metal oxide by heating under nitrogen atmosphere. Credit: UNIST
The novel material developed by the UNIST research team has exceptionally high CO2 adsorption capacity which could pave the way to save the Earth from CO2 pollution.
Nanoporous materials consist of organic or inorganic frameworks with a regular, porous structure. Because of their uniform pore sizes they have the property of letting only certain substances pass through, while blocking others. Nanoporous metal oxide materials are ubiquitous in materials science because of their numerous potential applications in various areas, including adsorption, catalysis, energy conversion and storage, optoelectronics, and drug delivery. While synthetic strategies for the preparation of siliceous nanoporous materials are well-established, non-siliceous metal oxide-based nanoporous materials still present challenges.
A description of the new research was published (Web) on May 7 in the Journal of the American Chemical Society. (Title: Nanoporous Metal Oxides with Tunable and Nanocrystalline Frameworks via Conversion of Metal-Organic Frameworks) This article will be also highlighted in the Editor's Choice of the journal Science.
Leading the research team was married couple Hoi Ri Moon and Sang Hoon Joo, both assistant professors at UNIST, who contributed to synthesizing nanoporous metal oxides and characterizing nanoporous materials respectively. Fellow authors include Tae Kyung Kim, Kyung Joo Lee, Jae Yeong Cheon and Jae Hwa Lee from UNIST.
The UNIST research team used MOFs based on aliphatic carboxylate ligands which are thermally less stable and much more labile than aromatic ligands. Specifically, the aliphatic ligand is adipic acid, which is a precursor for the production of nylon, and thus very important from an industrial perspective and low in price. During the thermolysis of a crystalline, aliphatic carboxylate ligand-based MOF (aph-MOF), the ligands were transformed into organic moieties via chemical decomposition, and were confined as vesicles in the solids.
The organic vesicles acted as self-generated porogens, which later were converted into nanopores; they also prevented aggregation of the metal oxide nanocrystals. Finally, upon thermolysis at higher temperature, the confined organic moieties evaporated, generating highly porous nanostructures comprising nanocrystalline metal oxides. The control of the retention time and the evaporation rate of the organic moieties in the host solid were critical for the successful formation of nanoporous metal oxides with nanocrystalline frameworks. The thermal treatments converted the Mg-aph-MOF into 3-dimensionally nanoporous MgO frameworks instead of discrete MgO nanoparticles embedded in a carbon matrix. Significantly, nanoporous MgO exhibited exceptional CO2 adsorption capacity (9.2 wt %) under conditions mimicking flue gas.
"I believe MOF-driven strategy can be expanded to other nanoporous monometallic and multimetallic oxides with a multitude of potential applications, especially for energy-related materials" said Prof. Moon. "Because of its high CO2 adsorption capacity, it will open a new way for environmental solutions."
"Various metal oxides converted from well-designed MOFs are being studied as fuel cell catalysts, also" said Prof. Joo, explaining his future research plan.