However, developing a facile and scalable process for synthesizing these bimetallic nanoparticles remains a considerable challenge. A recent study published in the journal Scientific Reports addresses this problem by proposing a sustainable and eco-friendly gas phase approach for synthesizing gold/cobalt (Au/Co) binary nanoparticles using continuous spark discharge plasmas.
Binary Nanoparticles (BNPs): Why are They Important?
Binary nanoparticles (BNPs) have recently gained popularity owing to their excellent photonic, electromagnetic, and catalytic capabilities in various applications, both in alloy and phase-segregated states. Binary nanoparticles are intriguing catalysts because the material's characteristics may be linked to the components that make up the nanoparticles.
Incorporating two or more metals into a single nanoparticle is extremely attractive since these materials may perform more than one function simultaneously.
Binary metallic nanoparticles are well-known for their capacity to catalyze a broad range of chemical processes, including the oxidation of carbon tetroxide and alcohol. The gold/cobalt (Au/Co) combination, a viable option for producing hydrogen from ammonia borane for hydrogen-powered automobiles, is a renowned example of binary nanoparticles as catalysts.
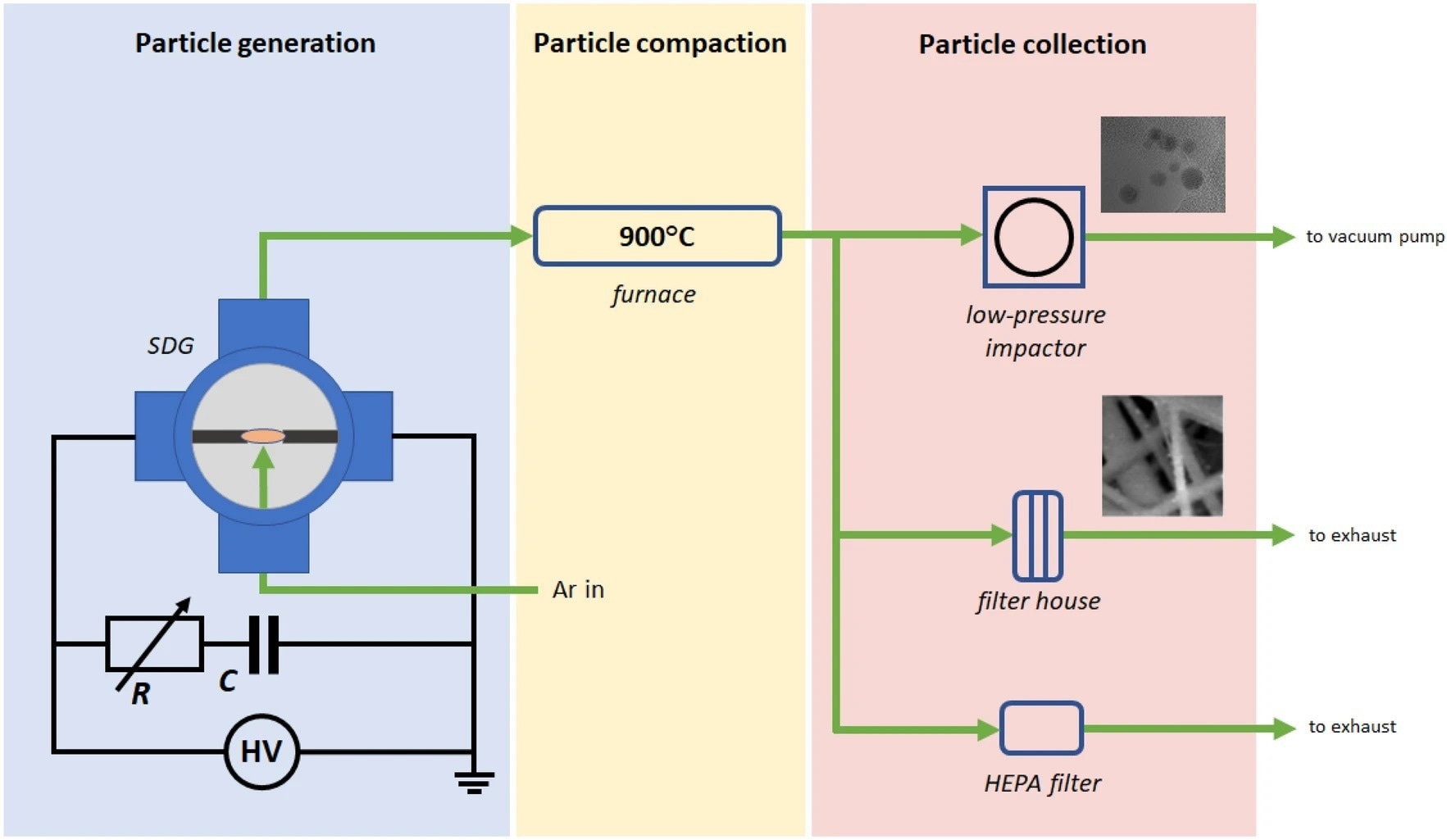
Figure 1. Schematic figure of the particle generation, collection, and sampling setup. © Villy, L. P., et al. (2022)
Challenges Associated with the Synthesis of Au/Co BNPs
Au/Co binary nanoparticles are often generated by chemical techniques, such as the concurrent reduction of gold and cobalt substrates.
The size- and composition control of the produced Au/Co BNPs is difficult because of the inherent utilization of multiple solvents and chemicals in typically quite complicated, multi-step procedures, which is a significant constraint for investigating their applications. In addition, the chemical byproducts of these reactions may be incorporated into the final colloidal solution, limiting the BNPs' applicability.
It is possible to exert far more control over Au/Co BNPs using gas-phase procedures, where particle generation is enhanced by the condensation and agglomeration of metallic ions and atoms in a gaseous or vacuum atmosphere. Au@Co and Co@Au core-shell BNPs with regulated size and structure can be made using the inert gas condensation approach and a super-saturated metallic vapor produced by sputtering a bulk target.
These methods provide considerable particle creation process control. However, they need complex equipment or high vacuum conditions, making their industrial expansion and real-world applications very difficult.
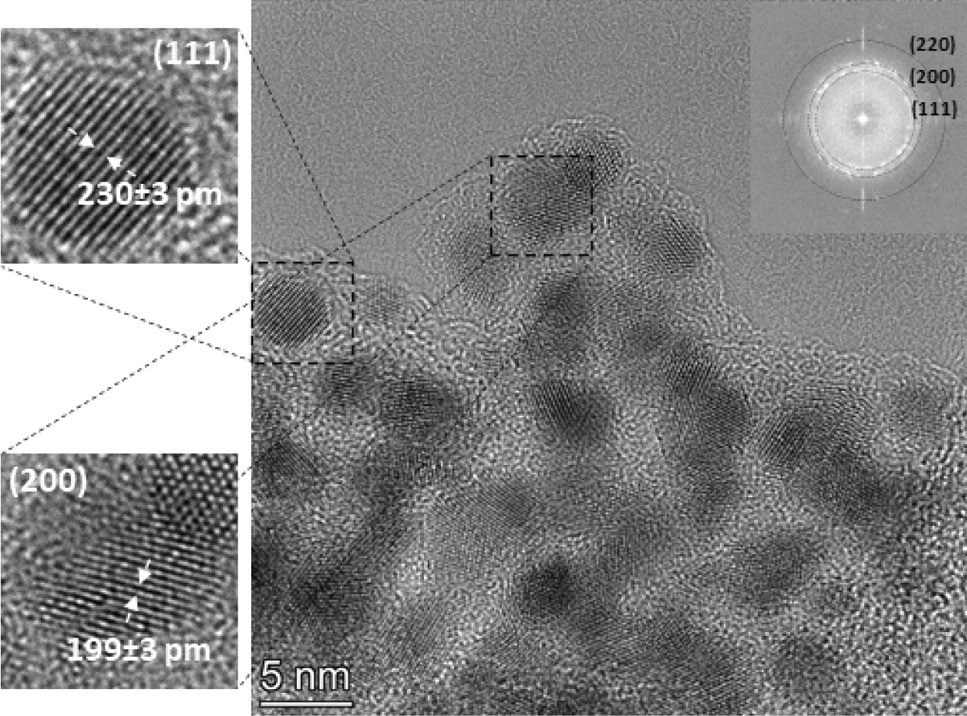
Figure 2. TEM micrograph of a typical aggregate of Au/Co BNPs (generated at 1.0 Ω circuit resistance), with close-ups of two different crystalline particles (on the left of the main image), showing the value of average lattice spacing obtained from the given region. The Fourier transform of the TEM micrograph of the whole aggregate is shown in the upper right corner of the image. © Villy, L. P., et al. (2022)
A Novel Method for Producing Au/Co Binary Nanoparticles
Spark ablation is a novel physical, gas-phase approach with considerable promise for adaptability and sustainability. It is based on a theoretically basic concept: the degradation of two conductive electrodes by microsecond-long, oscillating, repeating sparks at high current and high voltage.
Comparable to the gas-phase approaches described above, this method simply requires high-purity bulk electrodes and a regulated gaseous setting, allowing the production of highly pure BNPs. In particular, spark ablation does not need a vacuum environment, and its electrical application is simple, allowing for the scalable creation of nanoparticles.
In the current study, the researchers describe the production and characterization of Au/Co binary nanoparticles using spark ablation. In addition, they show the ability to tune the structure of Au/Co binary nanoparticles across a large range, as well as the ability to alter nanoparticle morphology.
The structure of as-prepared binary nanoparticles was examined using a high-resolution transmission electron microscope (HRTEM). Moreover, energy Dispersive X-Ray Spectroscopy (EDS) was used to produce the compositional profiles of the BNP samples.
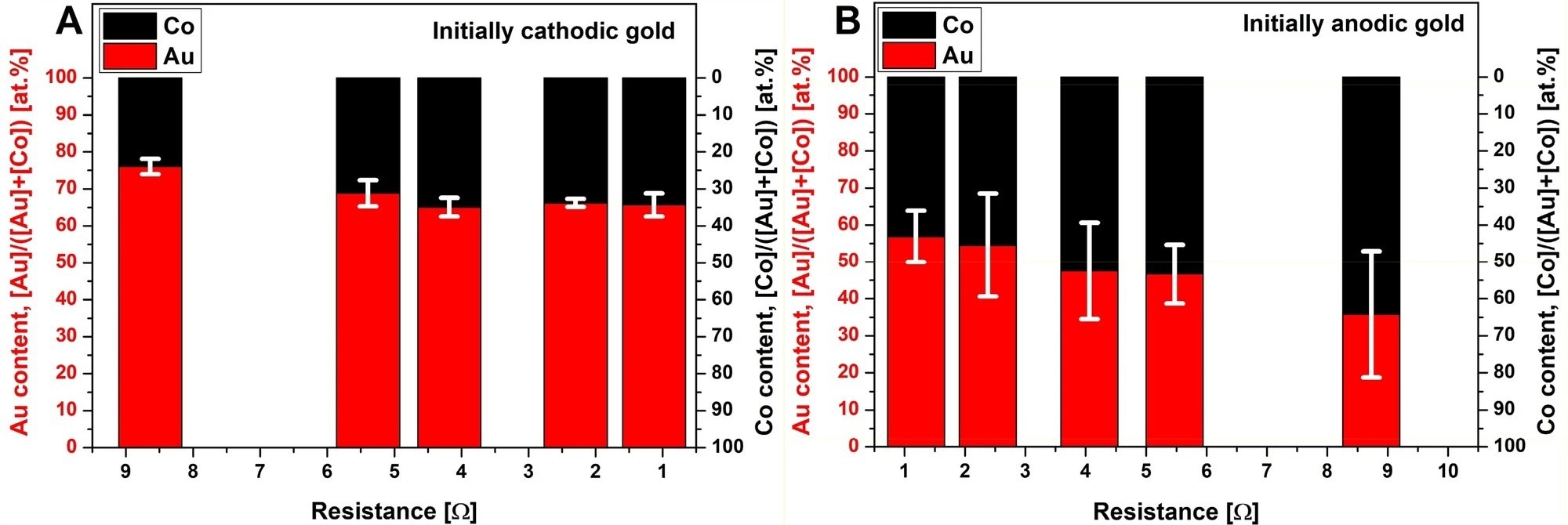
Figure 3. Variation of the composition of the Au/Co/CoO NPs obtained by ICP-MS, as a function of the total resistance of the discharge circuit when gold is initially cathodic (A) and anodic (B). Error bars indicate the uncertainty of the composition corresponding to a 90% confidence level. © Villy, L. P., et al. (2022)
Highlights and Key Developments of the Study
The gas phase production of Au/Co binary nanoparticles using electric spark discharge plasmas was shown in this research. The approach was demonstrated to produce gold-rich crystalline Au/Co particles immersed in a heterogeneous cobalt oxide matrix.
Thermal treatment of the as-produced nano aerosols can effectively modify the structure of the Au/Co binaries, favoring the development of polycrystalline Au/Co particles. The average elemental content of the nanoparticles is continually adjustable by adjusting the overall resistance of the discharge circuit and manipulating the spark current waveform.
Since the spark-based approach utilized in this work was shown to be reproducible even up to industrial scales, it is safe to conclude that these findings might help with the economic and durable production of Au/Co nanoparticles and their applicability in energy development and storage sectors.
Reference
Villy, L. P., et al. (2022). Continuous spark plasma synthesis of Au/Co binary nanoparticles with tunable properties. Scientific Reports. Available at: https://doi.org/10.1038/s41598-022-22928-0
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.