Researchers at the Department of Energy’s Pacific Northwest National Laboratory have announced a discovery. Despite forming in a perfectly hexagonal lattice, ice exhibits surprising flexibility and malleability, which accounts for the frequent entrapment of gas bubbles within ice. These findings are derived from the inaugural molecular-resolution observations of nanoscale ice samples frozen from liquid water, which were published in the journal Nature Communications.
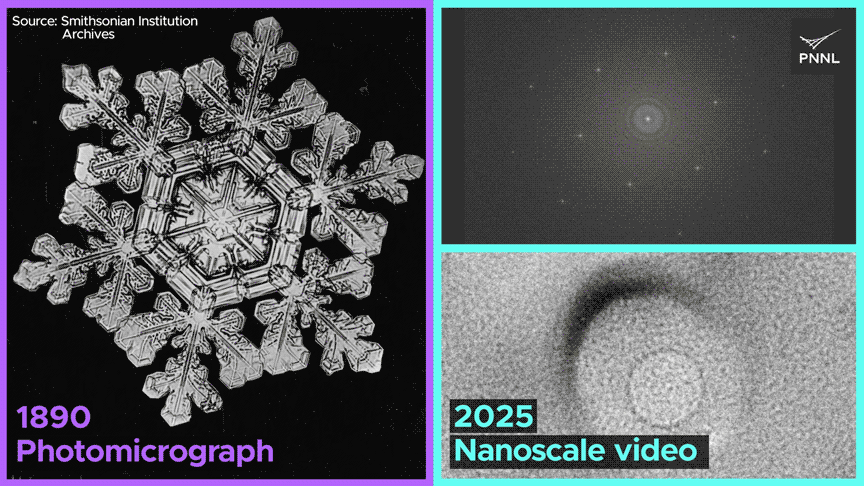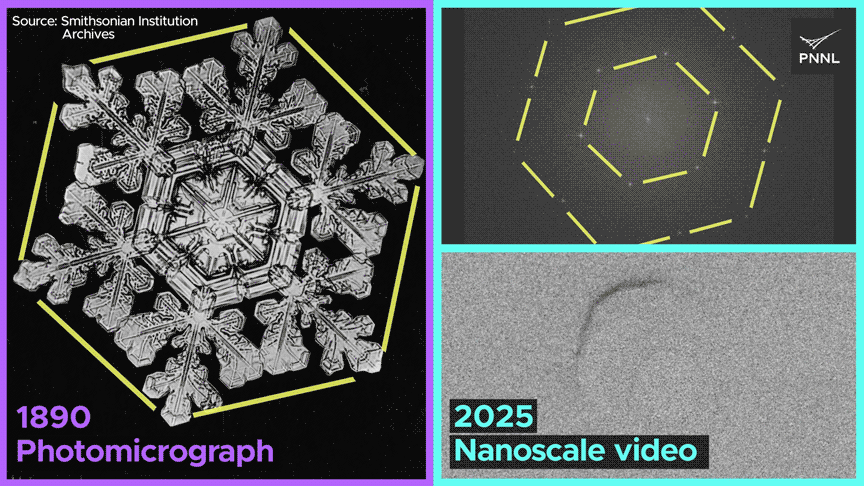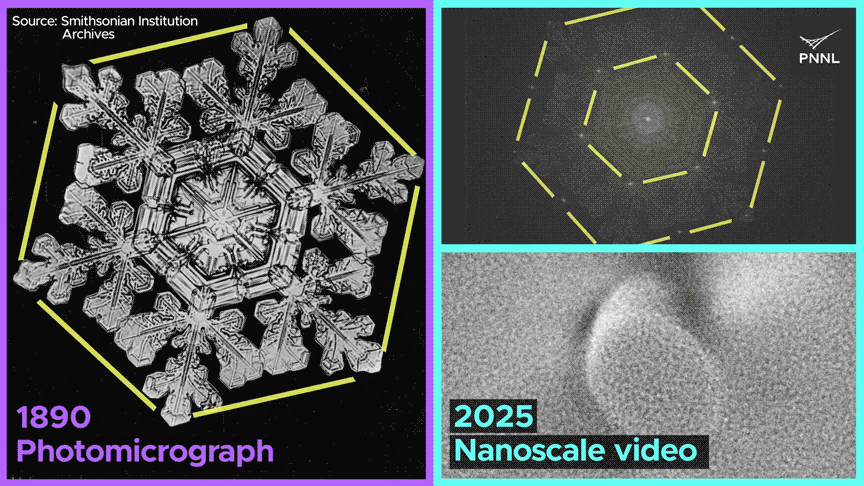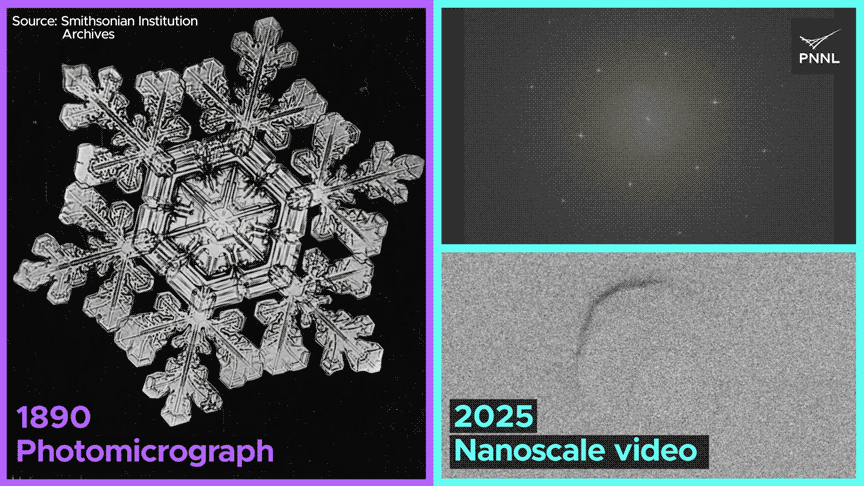 Watch how the same nanoscale forces shape both ice cubes and snowflakes. PNNL researchers just recorded the first-ever molecular-scale video of ice formed from liquid water over a century after this snowflake was photographed. Image Credit: Sara Levine | Pacific Northwest National Laboratory
Watch how the same nanoscale forces shape both ice cubes and snowflakes. PNNL researchers just recorded the first-ever molecular-scale video of ice formed from liquid water over a century after this snowflake was photographed. Image Credit: Sara Levine | Pacific Northwest National Laboratory
We observed dissolved gas not only generate cavities in ice crystals, but also migrate, merge with other gas bubbles and dissolve—behavior that is only possible due to the unusual nature of bonding in ice. This work opens up an entirely new opportunity to explore ice crystallization and melting behavior at scales unimaginable only a few years ago.
James De Yoreo, Study Principal Investigator and Battelle Fellow, Pacific Northwest National Laboratory (PNNL)
The study may have significant consequences for the preservation of cryogenically frozen biological tissue samples, predicting ice dynamics for the safety of aviation and vehicles, and comprehending glacier movement, among various other research domains.
There have been a lot of mysteries about ice. We want to understand how ice tolerates structural imperfections in the crystal and how trapped bubbles affect the mechanical properties of the crystal. Now we have a way to understand that.
Jingshan Du, Study Lead Author and Materials Scientist, Pacific Northwest National Laboratory
What’s New With Ice
No one has succeeded in directly observing water molecules transitioning from liquid to ice. This is because the methods employed by scientists to visualize individual atoms require extreme conditions, such as the use of high-energy radiation and the elimination of all air through vacuum sealing.
Although researchers have produced some images of ice at the molecular level, these images do not represent the typical freeze-thaw cycles experienced on Earth. Instead, they are created through a process of flash freezing that occurs directly from vapor to solid.
The research team placed liquid water between thin carbon membranes, which was the essential element facilitating this imaging advancement. Subsequently, they devised a novel technique known as cryogenic liquid-cell transmission electron microscopy to monitor the freezing process.
“The membranes protect the ice crystals from high vacuum and radiation, allowing us to acquire images with atomic-level information,” said Du.
The team observed the formation of gas bubbles, their movement through the lattice, their merging with other bubbles, and their subsequent dissolution.
The study indicated that when liquid water transitions into solid ice, the defects within its crystal structure or the presence of trapped gas bubbles do not induce significant strain on the ice crystal, which could lead to fracturing. It adjusts to the existence of these defects with remarkable ease in comparison to other solids, such as metals or minerals.
The characteristics of water's chemical bonds render it exceptionally flexible and malleable, even in its solid ice form. This recent observation, along with the essential fact that ice is less dense than liquid water, constitutes properties vital for sustaining life on Earth, particularly in marine environments.
The researchers conducted direct observations of the geometries and forces that influence ice crystal formation across all scales, including the development of snowflakes. Although snow originates from water vapor rather than liquid water, the same fundamental forces are in operation.
The scientists at PNNL worked in conjunction with researchers from Argonne National Laboratory and the University of Illinois-Chicago, who had employed machine learning to create a highly precise molecular dynamics model for ice. The comparisons made between experimental results and predictions from theoretical models affirmed that ice is distinct among solids in its ability to tolerate defects while maintaining the integrity of its crystal structure.
Why Trapped Air Bubbles in Ice Matter
While the PNNL team investigates ice dynamics at the nanoscale, other researchers find that air bubbles within glaciers significantly influence their behavior. Recently, scientists demonstrated that glaciers melt over twice as fast when they contain bubbles, in contrast to ice that is free of bubbles. Additionally, other scientists are seeking to prevent ice formation in sensitive tissue samples or on aircraft during flight.
The forthcoming phases of this study will involve examining melting processes and working with more complex samples, such as water containing dissolved substances.
Alongside Du and DeYoreo, PNNL researcher Ajay S. Karakoti; scientists Suvo Banik, Henry Chan, and Subramanian K. R. S. Sankaranarayanan from Argonne National Laboratory; Birk Fritsch and Andreas Hutzler from the Helmholtz Institute Erlangen-Nürnberg for Renewable Energy; and Ying Xia from the University of Washington also played a role in the research.
The study received funding from the DOE Office of Science, Basic Energy Sciences, Division of Materials Science and Engineering. The molecular dynamics simulations were funded by the Data, Artificial Intelligence, and Machine Learning at Scientific User Facilities program, which is part of the Digital Twin Project at Argonne National Laboratory.
A segment of the study was performed at the Environmental Molecular Sciences Laboratory, a scientific user facility at PNNL, as well as at the Molecular Foundry and the National Energy Research Scientific Computing Center, both of which are DOE-supported user facilities located at Lawrence Berkeley National Laboratory.
Why ice cubes trap air bubbles
Why do ice cubes so often trap air bubbles? Now we know, thanks to researchers at Pacific Northwest National Laboratory, who captured the first-ever nanoscale images of ice crystals formed from liquid water. Video Credit: Animation by Sara Levine | Pacific Northwest National Laboratory.
Journal Reference:
Chan, H., et al. (2025) Molecular-resolution imaging of ice crystallized from liquid water by cryogenic liquid-cell TEM. Nature Communications. doi.org/10.1038/s41467-025-62451-0