Jul 4 2008
In the emerging field of oxide electronics, electronic devices are based on oxide materials rather than conventional materials such as silicon. One of the most promising candidates for this application is the oxide semiconductor strontium titanate, SrTiO3. Now, researchers from RIKEN’s SPring-8 Center in Harima and their colleagues have uncovered fundamental properties of the electronic states in SrTiO3 that may be crucial to understanding the unusual electronic properties of this material.
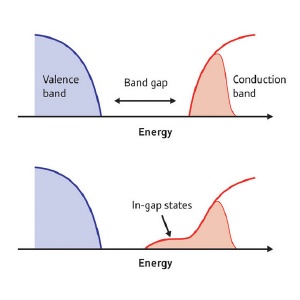 Electrons in SrTiO3. In regular semiconductors, excess electrons fill the conduction band (top; red shaded region). In SrTiO3, owing to an interplay between the electronic states of oxygen and titanium atoms, unusual electronic states form within the band gap region (bottom).
Electrons in SrTiO3. In regular semiconductors, excess electrons fill the conduction band (top; red shaded region). In SrTiO3, owing to an interplay between the electronic states of oxygen and titanium atoms, unusual electronic states form within the band gap region (bottom).
The strength of SrTiO3’s candidacy in oxide electronics is its versatility: it can be integrated with a number of related oxide materials to form complex electronic devices. “[In] particular, the interface between layers of SrTiO3 and other oxide materials has shown some novel properties, such as magnetism and superconductivity, whose precise origins are still under debate,” comments team-member Yukiaki Ishida. These properties are attributed to the way the material reacts to impurities that are intentionally introduced to provide a surplus of electrons.
Writing in the journal Physical Review Letters1, the researchers have uncovered the nature of the electronic states of SrTiO3. Unlike regular semiconductors, where all surplus electrons contribute to conductivity and fill the electronic states of the so-called conduction band, some of the electrons in SrTiO3 are not free to conduct and instead form localized electronic states within the band gap.
To investigate the origin of these so-called in-gap states, the team used x-rays from the SPring-8 synchrotron to force electrons out of SrTiO3 and measured their properties. Through fine-tuning of the x-ray energy, this emission is selective to a particular type of atoms, allowing for an unambiguous determination of the different atoms’ contribution to the electronic states.
Indeed, significant differences were observed between the electrons in the conduction band and those from the in-gap states. The electrons in the conduction band predominantly originate from Ti atoms, whereas those in the gap consist of electronic contributions from Ti as well as O atoms. In effect, the electrons from the oxygen ‘screen’ those from Ti, lowering their energy to below that of valence band electrons.
As Ishida asserts, this result is significant, as “previously the oxygen states were expected to play no role in the energetic states of SrTiO3, and people looked only at titanium.” The influence of oxygen on the electrons in SrTiO3 may explain some of the unusual properties of SrTiO3 so may lead to better control over these properties for enhanced oxide-electronic devices, for example in non-volatile information storage.
Reference
- Ishida, Y., Eguchi, R., Matsunami, M., Horiba, K., Taguchi, M., Chainani, A., Senba, Y., Ohashi, H., Ohta, H. & Shin, S. Coherent and incoherent excitations of electron-doped SrTiO3. Physical Review Letters 100, 056401 (2008).