Sep 17 2008
Our body is almost constantly being threatened by pathogens and cancerous cells that appear out of the blue. But the body puts up a fight: specialized cells in the immune system smuggle small molecules (granzymes) into cancer cells and those body cells that have fallen prey to viruses. The molecules then trigger off the diseased cells' built-in suicide program. There are two possible ways in which the granzymes gain entry into the cells under attack. Despite more than twenty years of research, however, it remained unclear as to which of these pathways is used to smuggle the lethal amount of granzymes into a cell. Scientists at the Max Planck Institute of Neurobiology have now shown that minute pores on the cell surface open the door to the granzymes for a short period of time. These results provide new prospects for improved methods of treatment of chronic virus infections and cancer. (PNAS, 2. September 2008)
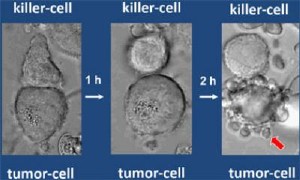 Granzymes going about their deadly work. A killer cell makes contact with a tumor cell (left) and detaches itself after one hour. After a further two hours, blisters appear (right, red arrow) on the surface of the cell that had been attacked. The tumor cells shrinks, dies and disintegrates. Image: Max Planck Institute of Neurobiology / Jenne
Granzymes going about their deadly work. A killer cell makes contact with a tumor cell (left) and detaches itself after one hour. After a further two hours, blisters appear (right, red arrow) on the surface of the cell that had been attacked. The tumor cells shrinks, dies and disintegrates. Image: Max Planck Institute of Neurobiology / Jenne
During our day-to-day life, we are rarely aware of the battles taking place in our own bodies. The body is almost always in a state of war against countless pathogens. And so, with every litre of blood that is pumped through our bodies, up to five billion white blood cells are sent out on patrol. Some of these cells react to pathogens by producing antibodies specially designed to attack precisely those pathogens that have been discovered. At the same time, they develop memory cells which recognize these pathogens immediately, should they attack anew.
In addition to these tacticians, a second group of white blood cells takes up arms against the enemy without further hesitation. The group consists of T-cells and killer cells that specialize in singling out body cells that have already been infected by viruses and tumor cells - swift action is therefore essential. However, these attackers also require tactics: in order to destroy a target cell, the attackers need to smuggle their weapons, known as granzymes, into the afflicted cell. Once inside, the granyzmes can carry out their deadly work by manipulating the diseased cell in such a way that it activates its suicide program. But how do the granzymes gain entry into the cell to begin with?
This is a question that scientists have been discussing for over twenty years. Granzymes were believed to gain entry into a cell either via pores or by membrane transport. T-cells and killer cells release a molecule called perforin which creates small holes in the cell membrane. Perforin might thus provide the granzymes with the openings they require. However, granzymes also bind to the surface of the attacked cells and are then internalized by membrane inversions and formation of small vesicles. Since the membrane pores created by perforin holes are fairly small and are quickly closed again by the besieged cell, most scientists favoured the latter theory that the granzymes' main mode of entry into a cell was membrane transport.
To determine what path the lethal dose of granzymes takes to enter a cell is no trivial matter. Such knowledge could be used to develop new therapeutic methods in the fight against viruses and cancer. Some twenty years on, scientists at the Max Planck Institute of Neurobiology now appear to have solved this question. Contrary to the generally accepted view, the membrane holes now seem to be the main point of entry for granzymes. The scientists proved this with artificially manipulated granzymes which no longer bound to membranes and which therefore could not enter the cell via membrane transport. "Interestingly enough, despite this restriction, the attacker cells were observed to be no less effective" declares Dieter Jenne. "We were also able to show that the pores are large enough to allow enough granzymes into the cell before the holes are resealed."
"The exciting thing about these results is not only that we have finally managed to answer a long-standing question", Florian Kurschus explains, "but that our granzyme variations, together with the knowledge that the membrane holes are the most important means of entry into the cell, can lead to improved therapeutic methods in the fight against viruses and cancer." High doses of artificially added granzymes can also damage healthy cells by entering them via membrane transport. The new granzyme variants do not accumulate in healthy cells, however, since they can only avail themselves of the pathway opened by T-cells or killer cells using perforin. In an infected cell that has been recognized by a T-cell or killer cell as an enemy, this door will be opened -wide enough for granzymes to enter and perform their deadly task.
Original work:
Florian Kurschus, Edward Fellows, Elisabeth Stegmann, Dieter Jenne
Granzyme B delivery via perforin is restricted by size, but not by heparan sulfate-dependent endocytosis
PNAS, September 2, 2008