Jan 29 2009
Photoelectron spectroscopy is a powerful technique for studying the composition and physical properties of a material. Despite its maturity, Yasutaka Takata from the RIKEN SPring-8 Center, Harima, and colleagues have discovered that there are still new things to learn about the physical phenomena on which it is based. They have observed an unexpected shift in the energy of electrons emitted by hard x-rays from the valence band of aluminum1-an effect that has implications for the interpretation of photoelectron data obtained at similar energies.
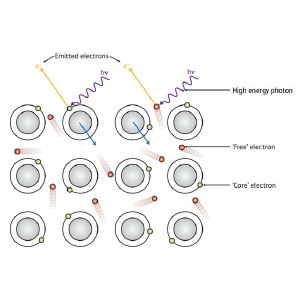 When a photon (hí) of sufficient energy hits a sample, it can excite the emission of electrons. For a metal, these electrons can come either from specific atoms (green circles) or from the sea of 'free' valence electrons (red) that extends over the whole sample.
When a photon (hí) of sufficient energy hits a sample, it can excite the emission of electrons. For a metal, these electrons can come either from specific atoms (green circles) or from the sea of 'free' valence electrons (red) that extends over the whole sample.
When a photon of light of sufficiently high energy is absorbed by a sample, it can cause the ejection of an electron. The kinetic energy the emitted electron depends on the energy of the light absorbed and the energy level the electron came from (Fig. 1). By using light with a well-defined energy and measuring the energies at which electrons emitted from a sample, it is possible to determine its chemical composition, how its atoms are bonded together, and its electronic structure, which determines its electronic, thermodynamic and optical properties.
When an electron is emitted from a sample, it transfers momentum to the sample, similar to the recoil that occurs when a bullet is fired from a gun. At the low energies at which photoelectron spectra are usually collected, the small mass of an electron relative to an atom makes this recoil negligible. But at higher energies, it becomes important.
In a previous study, Takata and colleagues observed a shift in the energy of electrons emitted from the inner shells of carbon atoms by high-energy, hard x-rays2. Their calculations showed that this shift could be explained, as expected, in terms of the loss of energy caused by the recoil of a high energy electron being ejected from a carbon atom.
What they found in their latest study, however, they did not expect. Analysis of high resolution hard-x-ray photoelectron spectra obtained aluminum revealed a shift in the energy of so-called 'free' electrons coming from its valence band. This is surprising because unlike core electrons, which are associated with individual atoms, valence band electrons belong to the sample as a whole. The recoil effect of these electrons should therefore be very small.
Such effects will need to be taken into account when interpreting hard x-ray photoelectron spectra. Takata suggests it might also provide a new tool for probing composite materials.
- Takata, Y., Kayanuma, Y., Oshima, S., Tanaka, S., Yabashi, M., Tamasaku, K., Nishino, Y., Matsunami, M., Eguchi, R., Chainani, A. et al. Recoil effect of photoelectrons in the Fermi edge of simple metals. Physical Review Letters 101, 137601 (2008).
- Takata, Y., Kayanuma, Y., Yabashi, M., Tamasaku, K., Nishino, Y., Miwa, D., Harada, Y., Horiba, K., Shin, S., Tanaka, S., Ikenaga, E., Kobayashi, K., Senba, Y., Ohashi, H. & Ishikawa, T. Recoil effects of photoelectrons in a solid. Physical Review B 75, 233404 (2007).