|
Researchers at Queen Mary, University of London and the Universite Paris Sud have used Accelrys' density functional theory (DFT) code CASTEP to study the formation of clay-polymer nanocomposite materials by self-catalyzed in situ intercalative polymerization.
|
|
|
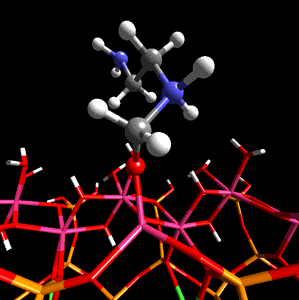
Figure 1. Methanal coordinated to aluminium in the tetradral layer of montmorillonite is activated towards nucleophilic attack by ethylenediamine.
|
Clay-Polymer Nanocomposites and Their Potential Applications
Clay-Polymer nanocomposite materials have recently attracted a great deal of attention as they offer enhanced mechanical and thermal properties as compared to conventional materials. Because of these enhanced properties they find application in the electronics, automobile, and furnishing industries.
Preparation of Clay-Polymer Nanocomposites by Intercalative Polymerization
One preparation of clay-polymer nanocomposites is in situ intercalative polymerization. This process involves mechanical mixing of the clay mineral with the required monomer. The monomer then intercalates within the interlayer and promotes delamination. Polymerization follows, initiated by a number of ways, to yield linear or cross linked polymer matrices. Often the clay mineral needs to be dispersed by a pre-swelling step of long-chain alkylammonium ion intercalation to aid exfoliation.
Self Catalyzed in situ Intercalative Polymerization
A recently discovered preparation, called 'self-catalyzed in situ intercalative polymerization', is similar to in situ intercalative polymerization, but differs in the fact that no pretreatment of the clay is required.
Modelling the Self Catalyzed in situ Intercalative Polymerization Process
Reporting in the Journal of the American Chemical Society, Stephen Stackhouse and Peter Coveney of Queen Mary, University of London, and Eric Sandre of Universite Paris Sud, describe how they used Accelrys' CASTEP to provide theoretical insight in to the mechanism of this novel process by determining the role played by the clay matrix.
The researchers first describe the work done on a specific example, that of methanal with ethylenediamine within a sodium montmorillonite interlayer, and then discuss the role that the clay structure may play in catalysis and the potential sources of Bronsted and Lewis acidity in sodium montmorillonite. The scientists then discuss previous in silico work done on clay minerals which leads to their reasoning behind their use of DFT - the fact that this method is particularly suited to the study of crystalline materials (e.g. clays and zeolites) when used in conjunction with periodic boundary conditions and a plane-wave basis set.
Using CASTEP to Optimize the Process
Using CASTEP as the optimization method on periodic model systems from Cerius2 4.2's structural database, Coveney and team showed that the proximity to isomorphic substitution sites within the silicate layers has a marked effect on the Bronsted acidity of the hydroxy groups. The hydroxy groups being further from the sites of the magnesium-for-aluminium substitution being more Bronsted acidic, although it was shown that it was energetically favorable for the to be nearer the substitution sites. The simulations also revealed that the monomer catalysis occurs at the mineral lattice edge. Here exposed Al3+ ions and hydroxy groups act as Lewis and Bronsted acid sites, respectively.
|