Chemists at the Nagoya University in Japan have synthesized novel cycloparaphenylene (CPP) chromium complexes and have shown the possibility of using them for obtaining monofunctionalized CPPs, which could help construct nanocarbons with unprecedented structures.
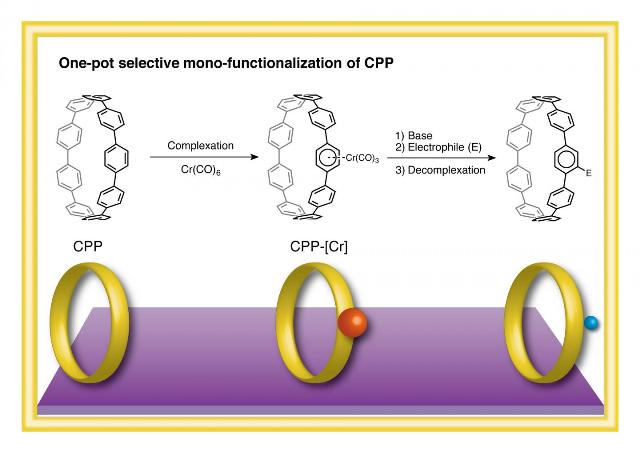 This image shows a one-pot selective monofunctionalization of CPP via a chromium complex. Credit: ITbM, Nagoya University
This image shows a one-pot selective monofunctionalization of CPP via a chromium complex. Credit: ITbM, Nagoya University
The team comprising Professor Kenichiro Itami, Natsumi Kubota and Yasutomo Segawa have synthesized the novel CPPs. The researchers are from the JST-ERATO Itami Molecular Nanocarbon Project and the Institute of Transformative Bio-Molecules (ITbM) at Nagoya University.
This project enables selective monofunctionalization of CPPs, which is the first time that this has been achieved. This could help lead to the construction of carbon nanotubes with unprecedented structures.
CPPs are the shortest segment of carbon nanotubes and they are made up of a chain of benzene rings. CPPs had been synthesized and isolated in 2008, and since then they have created significant interest in the fields of supramolecular chemistry and materials science.
Itami, along with his coworkers, applied the fundamental concepts of chromium arene chemistry, and conducted the first specific installation of a functional group on CPP. Prior to this, it had been very difficult to achieve as the CPP ring had multiple reactive arene sites. It is being considered that in the future, carbon nanotubes having new properties could be constructed using this method, by selectively installing and tuning the CPP’s functional groups.
The first synthesis, analysis and isolation of a CPP chromium complex have been demonstrated by this study. A one-pot access to monofunctionalized CPPs has been enabled by this achievement, and the outcome obtained is considered to be a significant advance in organometallic chemistry and CPP chemistry.
Arenes coordinate to transition metals. The corresponding metal complexes that form demonstrate different reactivities when considered with reference to the free arene. CPPs are made up of a chain of arenes, and when they react with chromium carbonyl, CPP’s first chromium complex was generated. An interesting part of the reaction was a CPP as a main product with one chromium moiety that was complexed to one arene on the ring’s outer side. This was confirmed using X-ray crystallography, high-resolution mass spectrometry and 1H NMR (nuclear magnetic resonance) spectroscopy.
Chromium arene chemistry is a well-established area and we decided to apply this organometallic method to synthesize the first CPP chromium complex.
Itami, the Director of the JST-ERATO project and the Institute of Transformative Bio-Molecules
As CPPs have a number of arene rings, we initially expected that chromium would form a complex with each arene ring. However, we were surprised to see that CPP reacted with chromium in a 1:1 ratio in all the conditions that we tried. Simulation of the molecular structure suggested that the first equivalent of chromium complexed to CPP lowers its reactivity, thus preventing the reaction with a second chromium moiety.
Segawa, a group leader of the JST-ERATO project
When the researchers found that a monometallic CPP complex could be realized, Itami's team analyzed the possibility of acquiring monofunctionalized CPPs from this CPP complex. The steps involved in achieving the monofunctionalized CPPs were described by Itami and Segawa.
This was not an easy task as chromium arene complexes are usually air and light sensitive, and CPP chromium complexes were no exception. But Natsumi worked persistently to obtain a pure crystal of the first CPP chromium complex.
Itami, the Director of the JST-ERATO project and the Institute of Transformative Bio-Molecules
We then performed the subsequent reactions in one-pot, to synthesize monofunctionalized CPPs after addition of base/electrophiles and removal of the metal from the CPP chromium complex.
Segawa, a group leader of the JST-ERATO project
Selective monofunctionalizations of CPPs are difficult to achieve due to the fact that all the carbon-hydrogen bonds that are on the arene rings are equivalent, chemically. Selective monofunctionalization is the process of installing a single functional group at a specific position on the arene ring. Usually, when direct functionalization of metal-free CPPs is done, they lead to uncontrolled multiple substitutions on the arene rings. CPPs are attractive for use as components in carbon nanotubes. However, till now there has not been any efficient method for obtaining directly functionalized CPPs.
"We were pleased to see that a functional group could be selectively installed on one arene ring via chromium coordination of CPPs," says Segawa. "As electrophiles, we utilized silyl, boryl and ester groups, which act as handles that can be easily transformed to other useful functionalities," he continues. Itami says, "We hope that this new approach evolves to become a valuable method to construct carbon nanotubes with unique structures and properties."
This study has been published online in the Journal of the American Chemical Society.
References