Dec 4 2008
Materials known as 'multiferroics' hold great promise as memory storage devices owing to coupling between their magnetic and electric properties. Alas, in the multiferroic materials known to date, this coupling typically is very weak and limited to low temperatures, hampering their uptake in commercial applications. Now, researchers from the RIKEN Advanced Science Institute, Wako, in collaboration with colleagues from the Japan Science and Technology Agency, the University of Tokyo and Tohoku University, have revealed strong multiferroic coupling in the oxide compound DyFeO3.
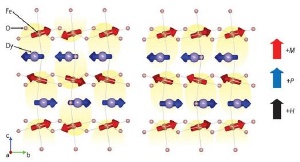 Multiferroic coupling in DyFeO3. In the absence of an external magnetic field, no net electric polarization (yellow areas) occurs: overall the electric polarization averages out. In the presence of a magnetic field H the Fe atoms rearrange their magnetic orientation M, leading to a net electric polarization P.
Multiferroic coupling in DyFeO3. In the absence of an external magnetic field, no net electric polarization (yellow areas) occurs: overall the electric polarization averages out. In the presence of a magnetic field H the Fe atoms rearrange their magnetic orientation M, leading to a net electric polarization P.
In multiferroic compounds ferromagnetism is coupled with ferroelectricity, a phenomenon where electric charges are separated in a material, such that an internal electric polarization is created. This coupling can be used for sensing applications, but also has potential in memory devices where data is typically stored as magnetic information and read out electronically.
Recently, some oxides of manganese, iron as well as others have been shown to possess strong coupling, but ferroelectricity in these materials is rather weak and only the electrical polarization can be switched by a magnetic field, and not vice versa—a showstopper for many applications. “Our goal is to find materials that show a full coupling between ferromagnetism and electric polarization, hopefully at room temperature,” says Yusuke Tokunaga, outlining the team’s research strategy.
As reported in Physical Review Letters*, the researchers have demonstrated that DyFeO3 shows large ferroelectric polarization combined with a strong multiferroic coupling. They found the origin of this behavior is the layered structure alternating between the dysprosium (Dy) and iron (Fe) layers, where the Fe atoms attract Dy atoms through their antiparallel magnetic orientation. In a zero magnetic field, the antiparallel pairs of Dy and Fe atoms cancel out the overall electric polarization.
Under the influence of a sufficiently strong magnetic field, however, the magnetic orientation of the Fe atoms rearranges slightly, which then leads to an electric polarization. As the electric polarization is a direct consequence of the magnetic structure, the multiferroic coupling is very strong—about two orders of magnitude larger than that of most other multiferroic materials.
Unfortunately, temperatures below -269 °C remain a necessity for the observation of this effect. Furthermore, the magnetic field required for the realignment of the magnetic orientation of the Fe atoms is relatively high. Nevertheless, Tokunaga is convinced that DyFeO3 represents a promising blueprint: “We believe DyFeO3 will serve as a template for materials with a large multiferroic coupling, even at higher temperatures.”
- Tokunaga, Y., Iguchi, S., Arima, T. & Tokura, Y. Magnetic-field-induced ferroelectric state in DyFeO3. Physical Review Letters 101, 097205 (2008).