Oct 21 2014
For the first time, the three dimensional structure of the protein that is essential for iron import into cells, has been elucidated. Biochemists of the University of Zurich have paved the way towards a better understanding of iron metabolism. The results also provide a basis for novel approaches to treat iron-related metabolic diseases.
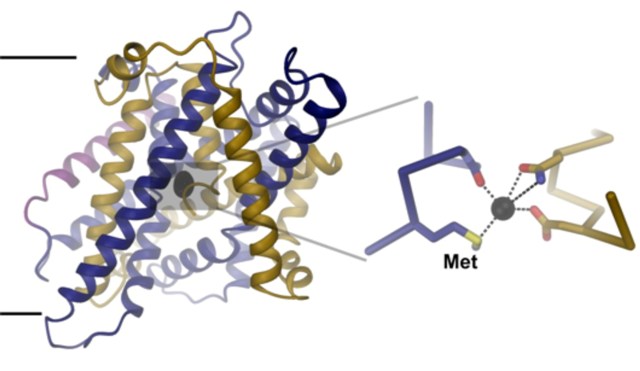 Structure of the iron transport-protein. The view is from within the membrane (indicated by black lines). A zoom into the iron binding-site (right) shows the interaction of the bound ion with conserved amino-acids. The methionine that is key for the ion selectivity is labelled (Met). (Image: UZH)
Structure of the iron transport-protein. The view is from within the membrane (indicated by black lines). A zoom into the iron binding-site (right) shows the interaction of the bound ion with conserved amino-acids. The methionine that is key for the ion selectivity is labelled (Met). (Image: UZH)
Iron is the most abundant trace element in humans. As a cofactor of certain proteins, it plays an essential role in oxygen transport and metabolism. Due to the major importance of iron in a wide variety of cellular processes, and the harm caused by its uncontrolled accumulation in the body, its uptake and storage is strictly regulated. In mammals, iron is imported into cells by the membrane transport protein DMT1. Mutations of DMT1, which affect its transport properties, lead to iron-related metabolic disorders such as anemia and the iron storage disease hemochromatosis.
Ines Ehrnstorfer, a PhD student in the group of Professor Raimund Dutzler at the Department of Biochemistry of the University of Zurich, and her colleagues, have determined the first structure of an iron transport protein. Their work was published in the scientific journal Nature Structural and Molecular Biology. Based on these results the researchers were able to explain why DMT1 binds the divalent metal ions iron and manganese (Fe2+ and Mn2+), but not calcium (Ca2+) – in spite of the latter being several orders of magnitude more abundant.
Moleclar basis for selective ion transport
To unravel the structural basis for this ion selectivity, Ines Ehrnstorfer has determined the structure of a close bacterial homologue of DMT1 by X-ray crystallography. The transport protein contains an ion binding site located at the center of the membrane that is composed of conserved amino acids. “One of these amino acids, a methionine, only interacts with transition-metal ions, but not with Ca2+”, explains Ehrnstorfer. The study also shows that mutations in the binding site weaken ion binding and transport in both the bacterial homologue and human DMT1.
“The results thus reveal how transition-metal ions such as iron are selectively transported across the membrane, and they provide a basis for the development of specific inhibitors of DMT1 for the treatment of iron storage diseases,” says the researcher. The project was funded by the Swiss National Science Foundation through the National Center of Competence in Research (NCCR) TransCure.
Literature:
Ines A Ehrnstorfer, et al. Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nature Structural and Molecular Biology, advanced online publication October 19 2014. Doi: 10.1038/nsmb.2904