Reviewed by Lexie CornerMay 28 2025
Researchers from the Complutense University, coordinated by Professor Nazario Martín, and the University of Malaga, led by Casado Cordón, have developed a new molecular model of bilayer graphene with controllable rotation. Published in Nature Chemistry, the study demonstrates how this rotational control can be used to regulate conductivity and explore semiconducting behavior.
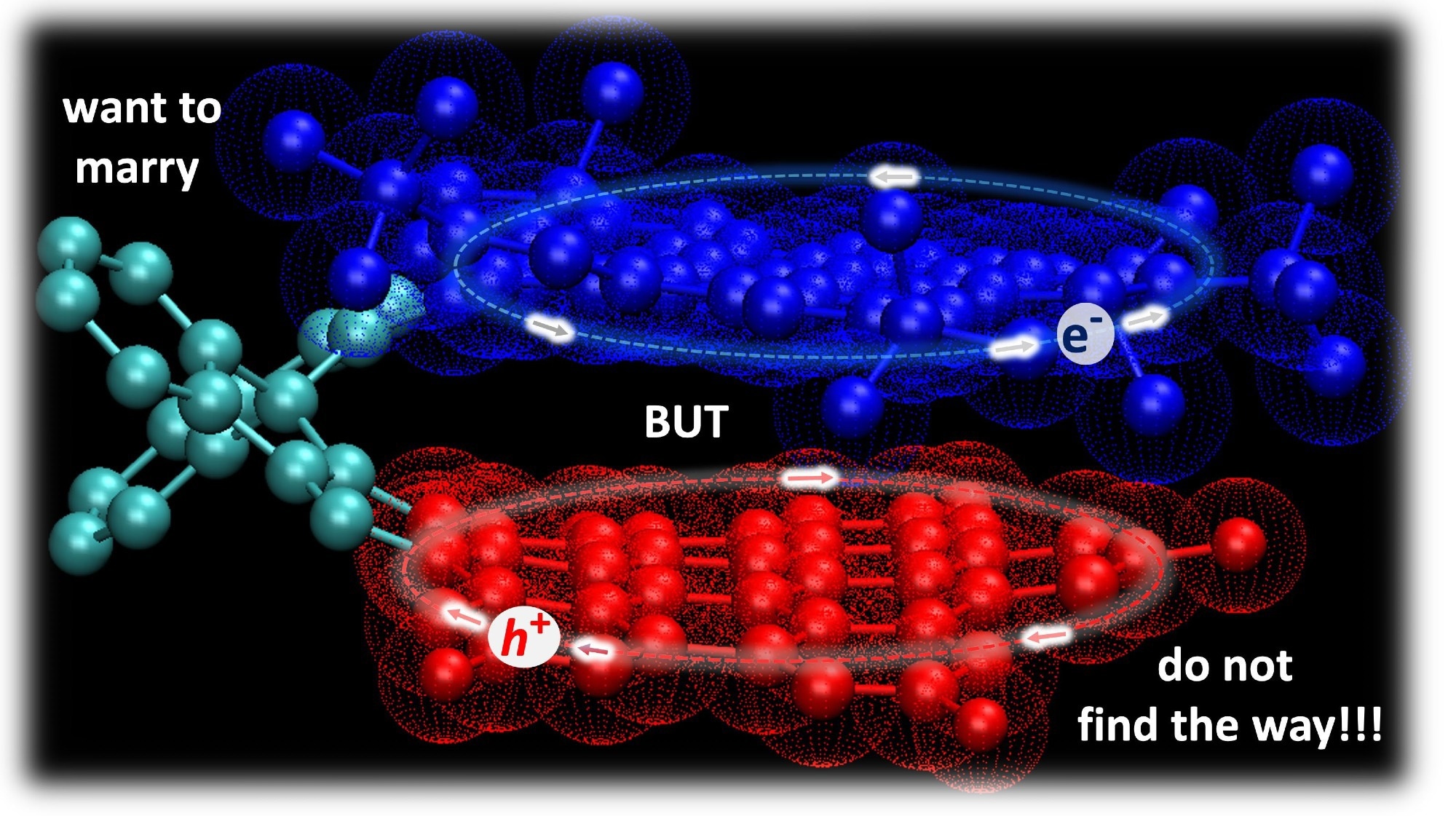 A new molecular model of bilayer graphene with higher semiconducting properties. Developed by scientists from the UMA and UCM, this material is capable of controlling the rotation between parallel segments and generating an efficient and durable ionic charge separation. Image Credit: University of Malaga
A new molecular model of bilayer graphene with higher semiconducting properties. Developed by scientists from the UMA and UCM, this material is capable of controlling the rotation between parallel segments and generating an efficient and durable ionic charge separation. Image Credit: University of Malaga
Juan Casado Cordón, Professor of Physical Chemistry at the University of Malaga, noted that graphene - a single layer of carbon atoms - has attracted major scientific interest over the past two decades due to properties like electrical and thermal conductivity, flexibility, and mechanical strength. He explained that these characteristics are further enhanced by combining two graphene layers to form bilayer graphene.
The result is a new molecular model that provides insight into the behavior of bilayer graphene systems.
By designing covalently bound molecular nanographenes, we can simulate the search for the magic angle between graphene-like sheets, which is where semiconductivity is achieved, a key property in, for example, the construction of transistors, the basic units of computers.
Juan Casado Cordón, Professor, Physical Chemistry, University of Malaga
Greater Efficiency and Durability
This UMA-developed model also enables the formation of ionic bonds between organic molecules, where one atom has a stronger influence in charge separation. This contrasts with most previous research on organic molecules, which has focused primarily on covalent bonding.
“Finding a metastable and lasting state of matter with electron transfer is a unique case between carbon molecules”, added Casado Cordón, who adds that this is a rare example of a 'quantum-mechanical' molecule with electrostatic bonding, which can be considered 'pre-quantum' or 'classical' due to its coulombic structure.
The findings support future work on artificial molecules that can replicate aspects of photosynthesis, converting light into electrostatic and then chemical energy. The bilayer nanographene developed in this study mimics the charge-transfer behavior of biological molecules involved in photosynthesis, opening the door to tailored photovoltaic applications.
Titled “Synthesis of zwitterionic open-shell bilayer spironanographenes,” the study took over six years and involved contributions from Samara Medina, who conducted the experiments, and Daniel Aranda, who carried out the theoretical modeling of charge transfer, both from the University of Malaga’s Department of Physical Chemistry.
The project also involved collaborations with international laboratories in Japan and Singapore, and with researchers at Complutense University of Madrid, led by Professor Nazario Martín, recipient of the ‘Enrique Moles’ National Research Award in Chemical Science and Technology.
Journal Reference:
Lión-Villar, J., et.al. (2025). Synthesis of zwitterionic open-shell bilayer spironanographenes. Nature Chemistry. doi.org/10.1038/s41557-025-01810-2