Jul 8 2008
Scientists at CNRS-affiliated laboratories(1) in Bordeaux, Lyon and Paris have provided the first proof that amorphous materials, also known as soft glasses, deform and flow through a collective movement of their particles. These materials (which include chocolate mousse, shaving cream, mayonnaise, metallic glasses, granular materials and mud) are amorphous solids, in other words, they are resistant like solids but, like liquids, lack a crystalline structure. This discovery, published in the July 3, 2008 issue of the journal Nature, should make it possible to better understand deformation and fracturing in metallic glasses(2) and the spreading of thin layers of fragile materials (such as face creams) used in the cosmetics, food-processing and lubrication industries.
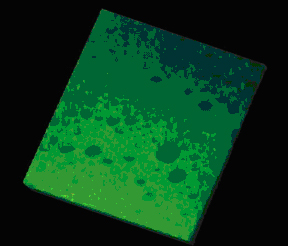 Cross-section of a dense emulsion, made up of numerous oil droplets of various sizes. © Julie Goyon, LOF, Bordeaux (This image can be obtained from the CNRS photo library, [email protected]).
Cross-section of a dense emulsion, made up of numerous oil droplets of various sizes. © Julie Goyon, LOF, Bordeaux (This image can be obtained from the CNRS photo library, [email protected]).
An amorphous solid is a liquid that does not flow: its atomic structure is disordered like that of a liquid but it is rigid and holds its shape like a solid. Amorphous materials include silica glass and a multitude of other materials of different origins, such as soft glasses (like concentrated emulsions, mousses and colloidal glasses). They are often found on our kitchen tables – as mayonnaise, chocolate mousse or ketchup – or in our bathrooms in the form of various gels or beauty creams. These soft glasses are actually capable of flowing if forced: mayonnaise looks solid in a jar, but can be spread with a knife, just like liquid honey.
The nature and origin of this amorphous state, however, continues to be a scientific challenge for researchers. Do these materials deform like related crystalline solids? Or do they flow like the liquids that share their structure? The dual nature of these materials is of great value from an industrial point of view, something which led CNRS and Rhodia to take an interest in them in the joint research unit “Laboratoire du Futur.” Progress has been made on multiple fronts in the last few years. Experimental and theoretical work on amorphous solids at rest has made it possible to better understand the paradoxical nature of the rigid structure of these materials: On the atomic level, their particles jam collectively, which causes their organizational structure to come to a nearly complete standstill. The link with their flow behavior, however, had not been previously established.
Scientists carried out a series of experiments using “microfluidic"(3) techniques that allowed them to observe the flow of highly concentrated emulsions in microchannels of various widths. These substances are made up of a very concentrated assemblage of silicon oil droplets suspended in a solvent(4) that makes it possible to view the interior of the emulsion under a microscope. This is a typical example of a soft glassy material: the droplets are completely disordered yet the emulsion does not flow unless a sufficiently strong force is applied. By analyzing the flow of this material in channels of micrometer scale width, the researchers were able to identify that the intrinsic flow properties of the material (its “rheology”) depend on its confinement, in other words, the fact that it is being forced to flow in a narrow channel. Surprisingly, in certain situations, the material appears to be even more fluid when it is confined.
Detailed analysis of these properties reveals that large-scale movements of flowing particles take place collectively (here, the particles are drops of oil that move as a block). This cooperativity effect is, however, very different from that which has been observed in the collective dynamic of amorphous materials that are not flowing, thus opening new theoretical questions.
This study clarifies the flow properties of amorphous solids and should help improve modeling of these complex phenomena. The collective nature identified in the flow differs from usual proposed descriptions. One objective is to be able to better predict and understand the tribological properties of thin films as well as the way glass breaks.
Notes :
- Laboratoire du Futur (LOF) (Rhodia/CNRS/Université Bordeaux 1), Laboratoire Gulliver (Ecole supérieure de physique chimie industrielle de Paris/CNRS), Laboratoire de physique de la matière condensée et nanostructures (LPMCN) (CNRS/Université Lyon 1) and Physics Department, Technical University of Münich, Institut Navier (a federated research structure covering three research units in the field of Mechanics and Physics of Materials and Structures, with each unit belonging to one or more of the following establishments: CNRS/École Nationale des Ponts et Chaussées (ENPC)/Laboratoire Central des Ponts et Chaussées (LCPC)/Université de Marne la Vallée (UMLV)).
- Metallic glasses are new amorphous materials composed of metallic alloys with disordered atomic structures.
- These techniques involve making fluids flow in channels whose width is on the order of several micrometers.
- Made up of a mixture of water and glycerin.
References :
- Spatial cooperativity in soft glassy flows », Julie Goyon, Annie Colin, Guillaume Ovarlez, Armand Ajdari & Lydéric Bocquet, Nature, vol: 454, Issue: 7200; pages: 84-87 (2008); DOI: 10.1038/nature07026