Jun 15 2013
Cheaper clean-energy technologies could be made possible thanks to a new discovery. Research team members led by Raymond Schaak, a professor of chemistry at Penn State, have found that an important chemical reaction that generates hydrogen from water is effectively triggered -- or catalyzed -- by a nanoparticle made of nickel and phosphorus, two inexpensive elements that are abundant on Earth.
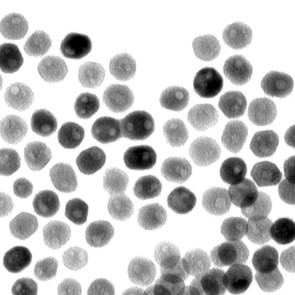 A transmission-electron microscope image of a collection of quasi-spherical nickel phosphide nanoparticles. A team led by Raymond Schaak of Penn State has found that these nanoparticles can catalyze an important chemical reaction that generates hydrogen from water. Image: Eric Popczun
A transmission-electron microscope image of a collection of quasi-spherical nickel phosphide nanoparticles. A team led by Raymond Schaak of Penn State has found that these nanoparticles can catalyze an important chemical reaction that generates hydrogen from water. Image: Eric Popczun
The results of the research will be published in the Journal of the American Chemical Society. More details and two photos are online at http://science.psu.edu/news-and-events/2013-news/Schaak6-2013.
Schaak explained that the purpose of this nanoparticle is to help produce hydrogen from water -- a process that is important for many energy-production technologies including fuel cells and solar cells. "Water is an ideal fuel, because it is cheap and abundant, but we need to be able to extract hydrogen from it," Schaak said. Hydrogen has a high energy density and is a great energy carrier, Schaak explained, but it requires energy to produce.
To make its production practical, scientists have been hunting for a way to trigger the required chemical reactions with an inexpensive catalyst. Platinum works, but it is expensive and relatively rare, so Schaak and his team have been searching for alternative materials. "There were some predictions that nickel phosphide might be a good candidate, and we already had been working with nickel phosphide nanoparticles for several years," Schaak said. "It turns out that nanoparticles of nickel phosphide are indeed active for producing hydrogen and are comparable to the best known alternatives to platinum."
To create the nickel phosphide nanoparticles, team members began with metal salts that are commercially available. They then dissolved these salts in solvents, added other chemical ingredients, and heated the solution to allow the nanoparticles to form. The researchers were able to create a nanoparticle that was quasi-spherical -- not a perfect sphere, but spherical with many flat, exposed edges. "The small size of the nanoparticles creates a high surface area, and the exposed edges means that a large number of sites are available to catalyze the chemical reaction that produces hydrogen," Schaak explained.
Team members at the California Institute of Technology then tested the nanoparticles' performance in catalyzing the necessary chemical reactions. They found that, not only were the chemical reactions happening as they had hoped, they also were happening with a high degree of efficacy.
"The goal now is to further improve the performance of these nanoparticles and to understand what makes them function the way they do," Schaak said. "Our team members believe that our success with nickel phosphide can pave the way toward the discovery of other new catalysts that also are comprised of Earth-abundant materials. Insights from this discovery may lead to even better catalysts in the future."
In addition to Schaak and Lewis, other researchers who contributed to this study include Eric J. Popczun, Carlos G. Read, Adam J. Biacchi and Alex M. Wiltrout from Penn State; and James R. McKone from the California Institute of Technology.
The research was funded by the U.S. National Science Foundation and the U.S. Department of Energy. The team has filed a patent application.